- 中文
- 搜索
打造專業(yè)的醫(yī)療解決方案團隊,提高新型設(shè)備、新藥研發(fā)的國際注冊進程。我們致力于消除溝通障礙,將最先進的醫(yī)療技術(shù)的聲音傳達到全世界的醫(yī)生和患者手中。

IEC 報告、X 光機、MRI、CT、超聲儀等大型醫(yī)療器械的產(chǎn)品說明書及注冊材料,輻射學(xué)、核醫(yī)學(xué)、實驗室及臨床各科診斷及檢測設(shè)備,血液生化檢測、ELISA(酶聯(lián)免疫吸附實驗)試劑盒、基因芯片、蛋白芯片技術(shù)等。
藥理學(xué)、毒理學(xué)、生藥學(xué)、藥物化學(xué)、藥物分析學(xué)、藥劑學(xué)、制劑處方及工藝、原輔料來源及質(zhì)量標(biāo)準(zhǔn)、藥品檢測報告、生物制藥、新藥急性毒性、慢性毒性試驗、新藥臨床試驗、新藥報批資料全套的整理和翻譯,研究者手冊等。
食品、飲料、化妝品、護膚品、以及保健品等。
獸醫(yī)學(xué)、畜牧學(xué)、寵物馴養(yǎng)、獸用醫(yī)療器械、獸用藥物、貓糧、狗糧等資料。
生命科學(xué)領(lǐng)域國際學(xué)術(shù)會議、研討會演講 ppt 制作和翻譯、視頻影像資料,大會日程及紀(jì)錄翻譯等
醫(yī)學(xué)科普公共信息類;病歷及住院記錄;醫(yī)藥網(wǎng)站、宣傳資料等;醫(yī)學(xué)網(wǎng)站及數(shù)據(jù)庫本地化;醫(yī)學(xué)軟件使用手冊及界面漢化等

eCTD 是英文 Electronic Common Technical Document 的簡稱,eCTD 由 CTD 發(fā)展而來。隨著醫(yī)藥研發(fā)的演進,注冊文件遞交的方式,總共經(jīng)歷了四種變化:
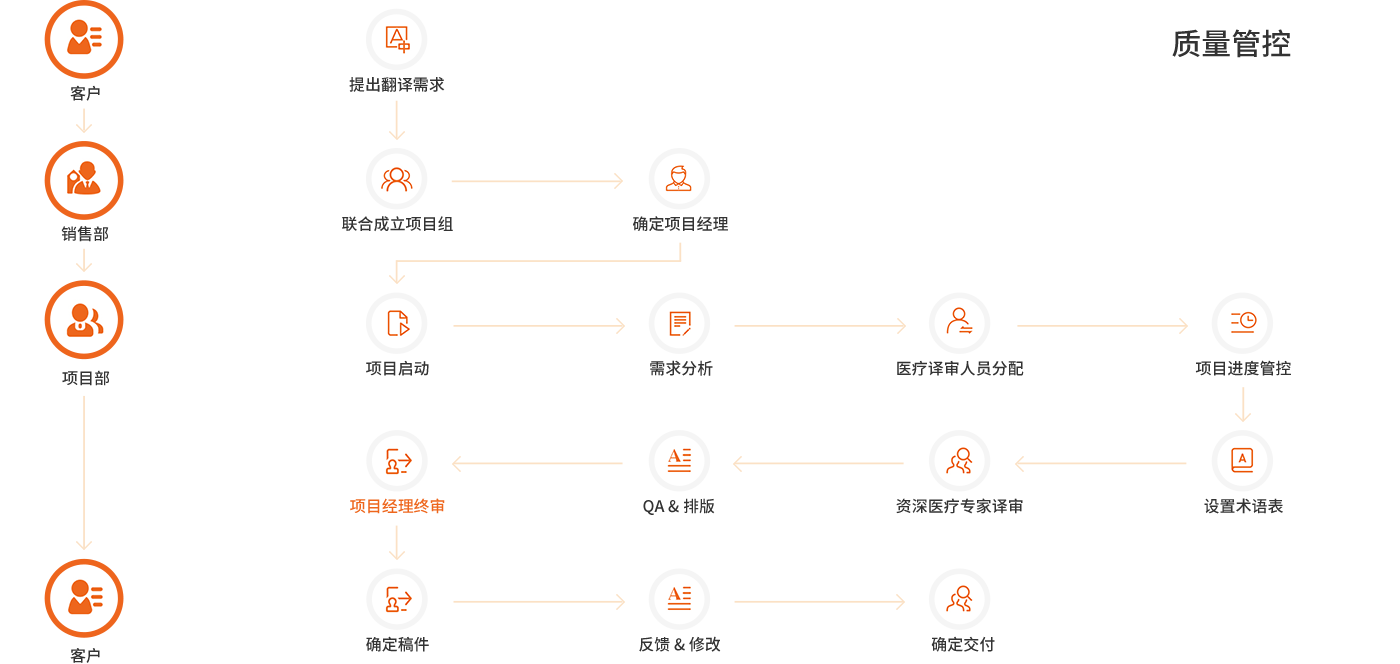
我們的生命科學(xué)翻譯團隊的譯員和審校均具有 5 年以上從業(yè)經(jīng)歷,經(jīng)過多年的歷練和自身刻苦鉆研,具備較高的生命科學(xué)領(lǐng)域的專業(yè)知識水平。
對于行業(yè)專業(yè)術(shù)語具有很強的敏感性。為客戶提供的翻譯成果專業(yè)、準(zhǔn)確。
· 已通過 ISO9001 質(zhì)量管理體系、ISO17100 國際翻譯服務(wù)流程標(biāo)準(zhǔn)及 ISO27001 信息安全管理體系認證
· 具備豐富的行業(yè)經(jīng)驗,譯者的專業(yè)醫(yī)學(xué)知識、語言精準(zhǔn)度及細致性都很高
· 熟悉全球市場的地方性法規(guī)要求,能提供準(zhǔn)確、及時且合規(guī)的譯文
· 響應(yīng)迅速,按時交付,充分配合客戶的產(chǎn)品上市時間
